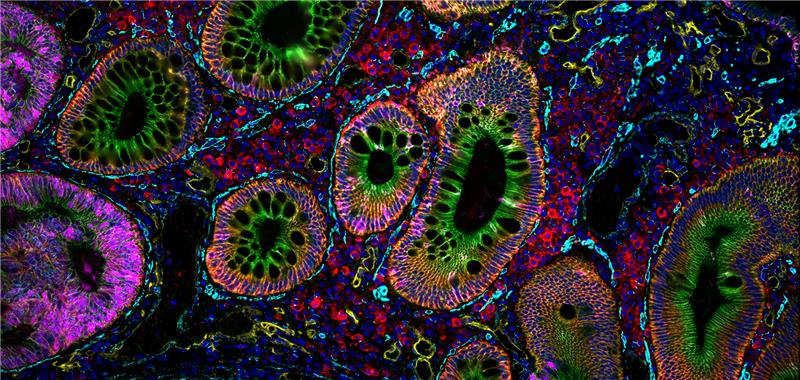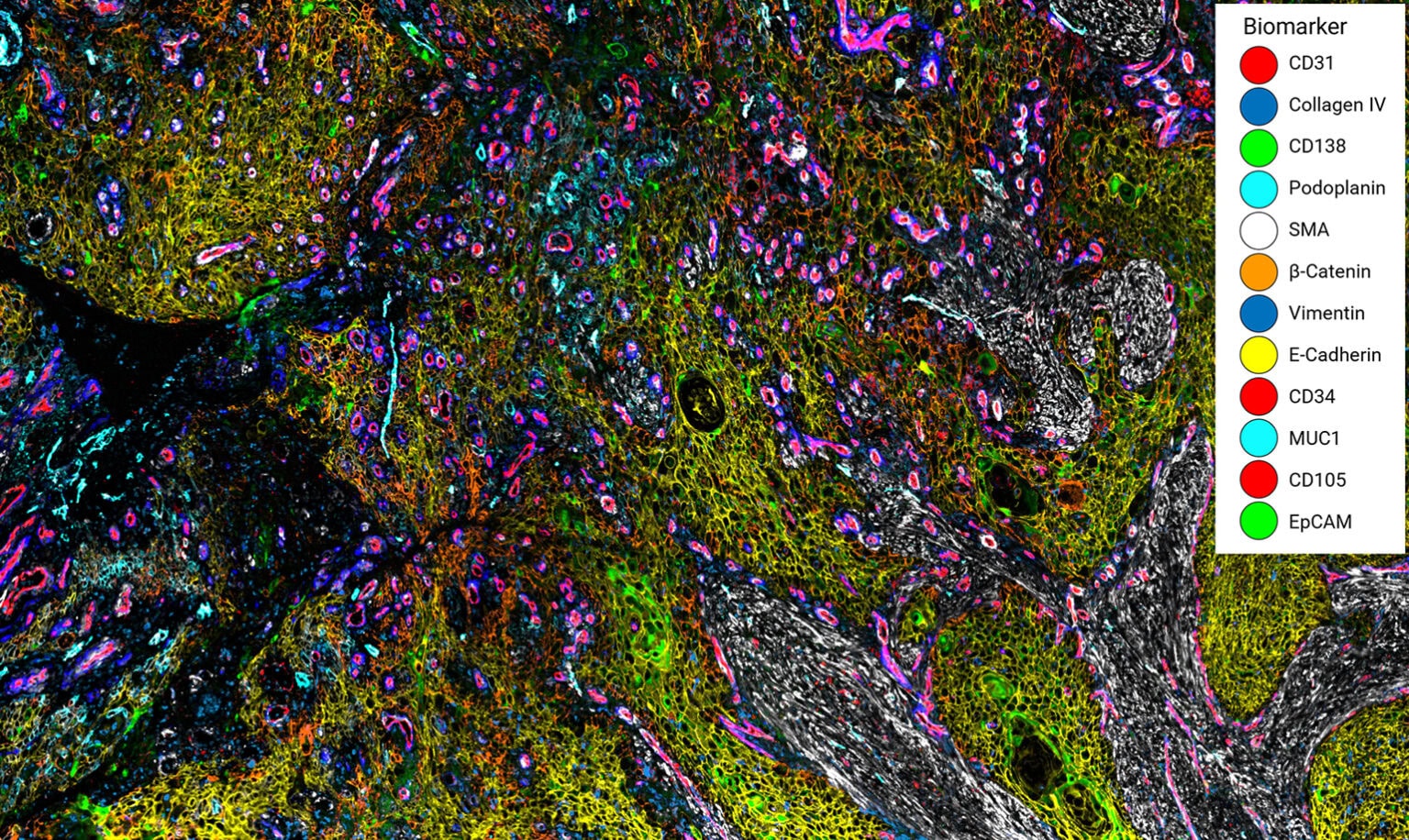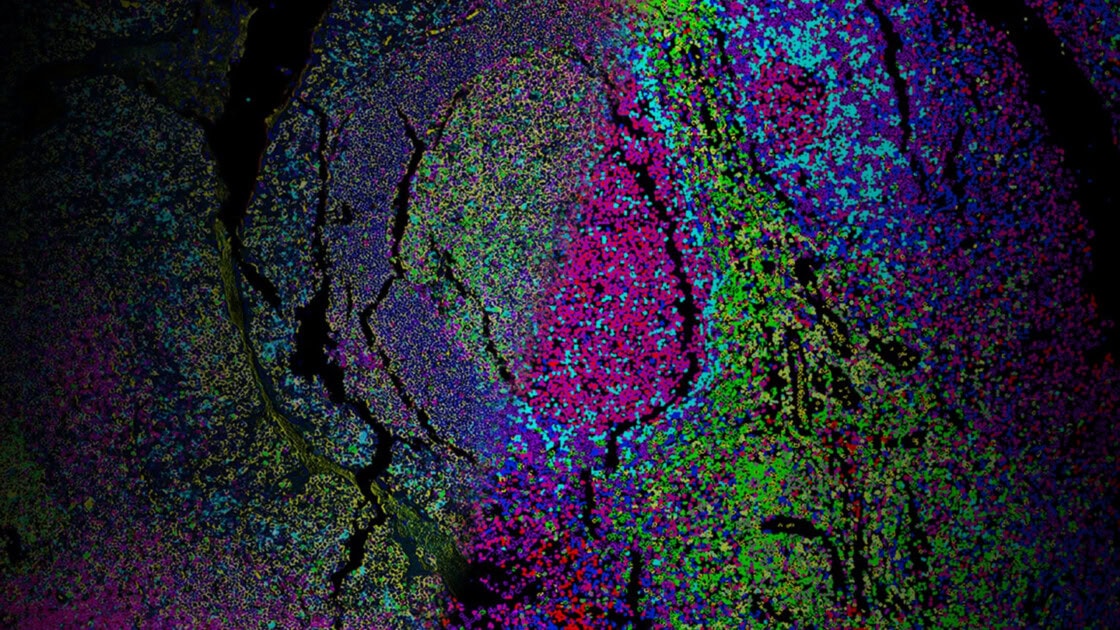
Enabling Precise Single-Cell Segmentation
The Cell Boundaries Assay Kit delivers fluorescently labeled antibodies for cell boundary staining optimized by our robust validation workflow, enabling clear delineation of individual single cells within intact tissue architecture.
By improving segmentation accuracy, the kit ensures each protein signal is correctly assigned to its cell of origin, resulting in cleaner, more reliable data for cell type classification, spatial mapping, and neighborhood analysis. The panel can be expanded by pairing with additional VistaPlex Multiplexing Assay Kits or your markers of choice.
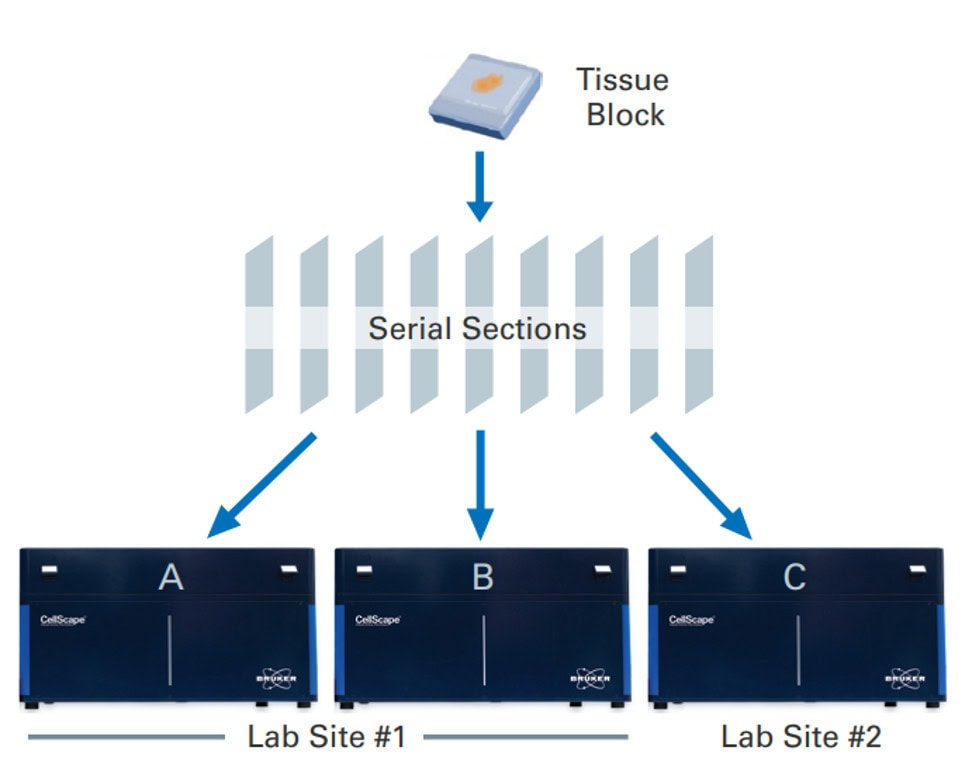
The VistaPlex Cell Boundaries Assay Kit enables automated staining and imaging of 10 human FFPE samples for 4 cell segmentation biomarkers using the CellScape Precise Spatial Proteomics platform. An online protocol is available to guide consistent and reliable execution of the multiplexed assay.

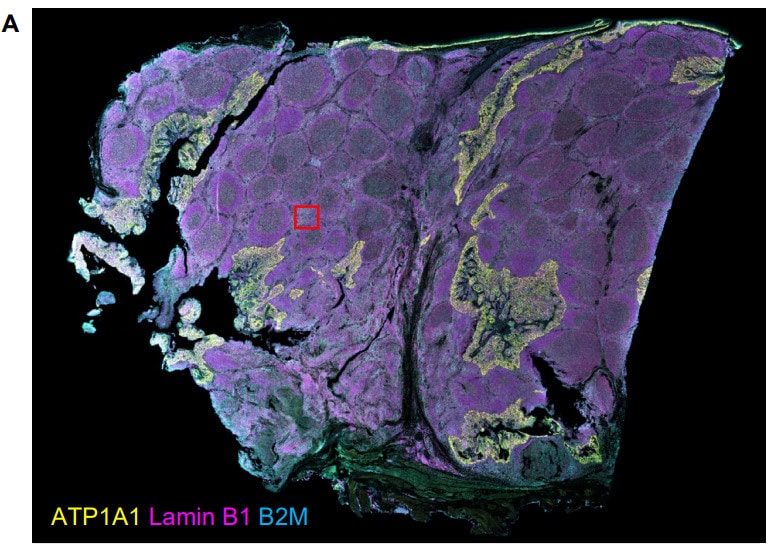
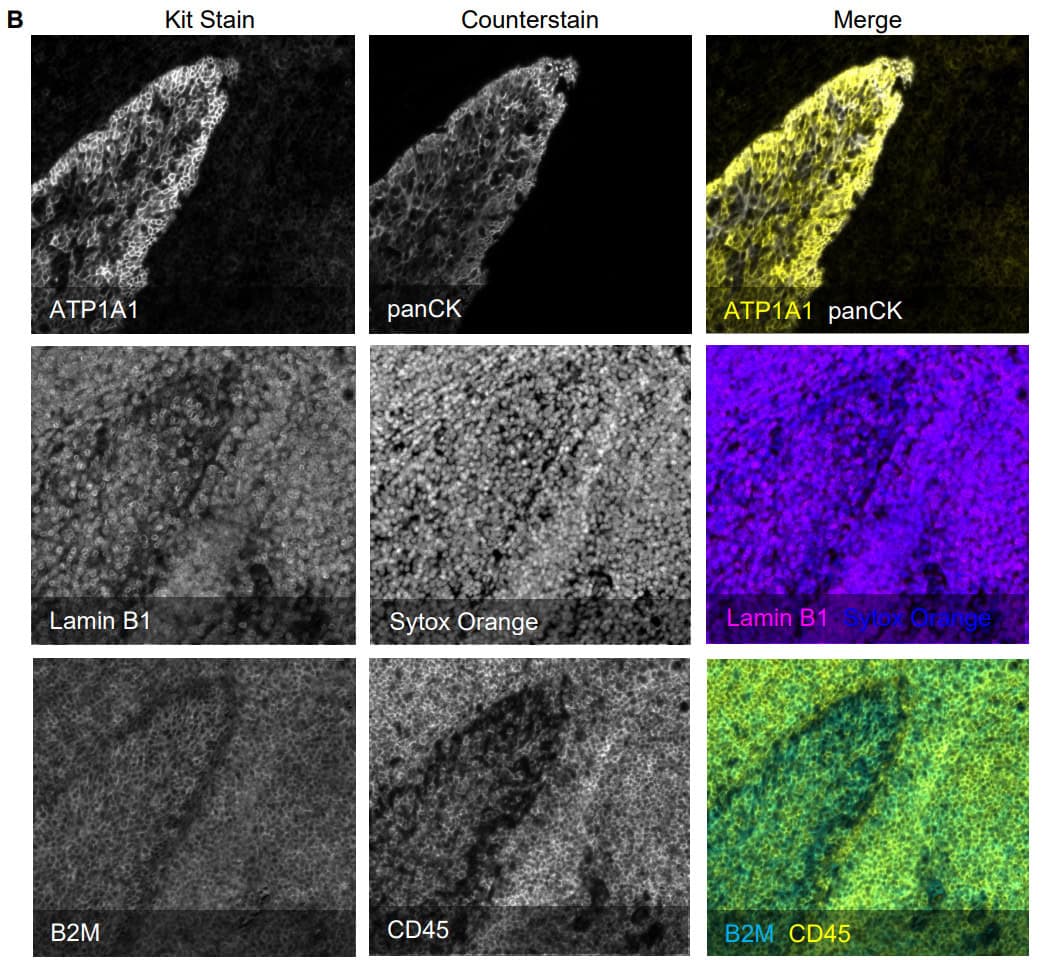
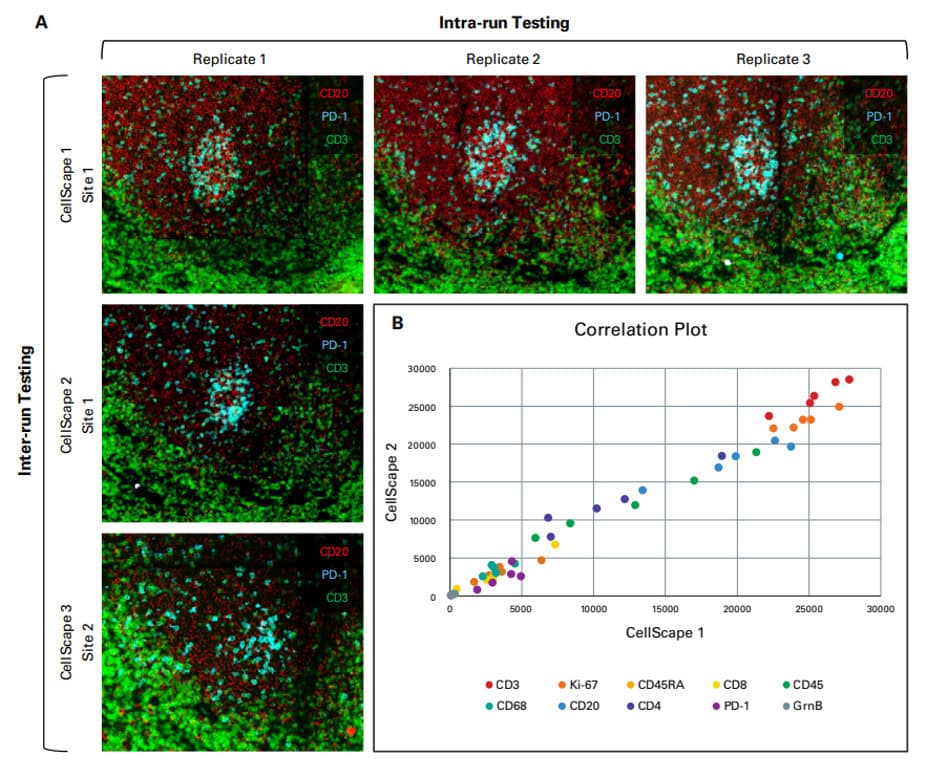
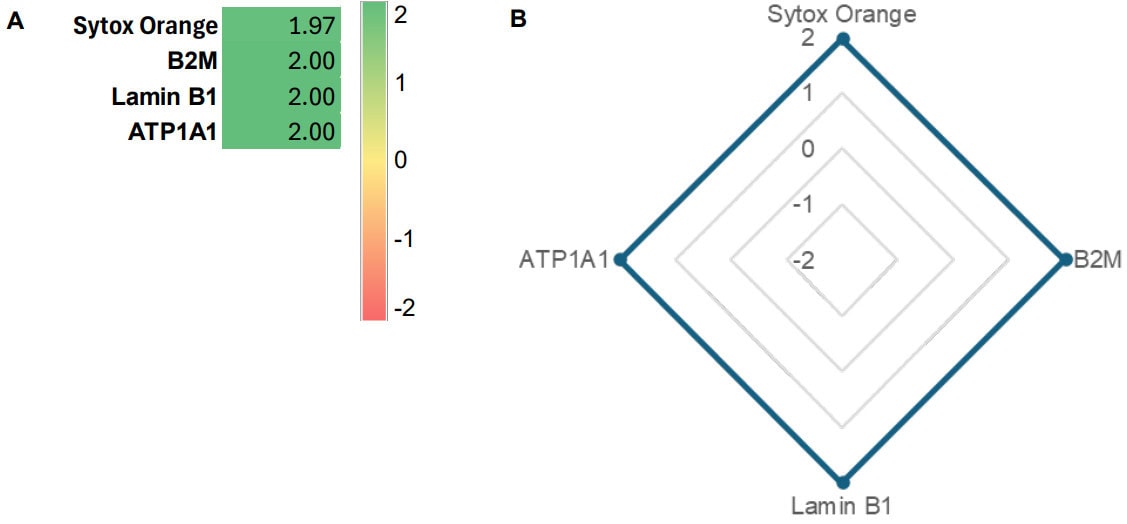
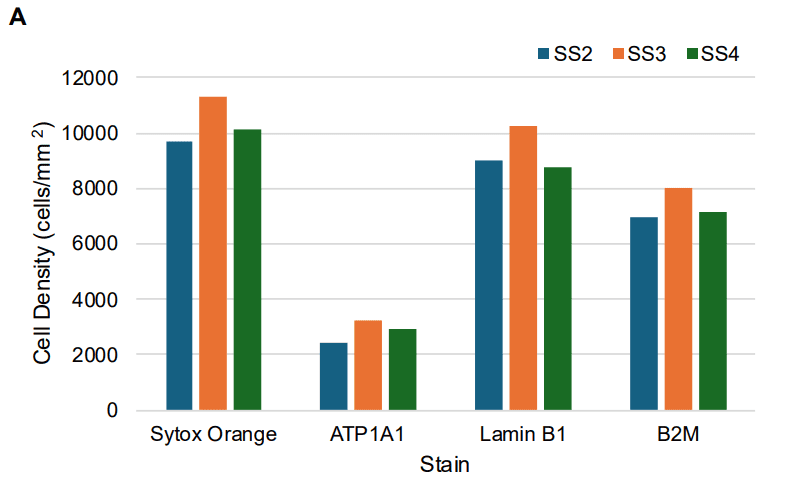
Validation is performed on human FFPE tonsil as a positive control, as well a multiple fundamental disease states.
Request a Quote
Contact our helpful experts and we’ll be in touch soon.

Related Resources