Fundamental Spatial Immunophenotyping of the Immune Microenvironment
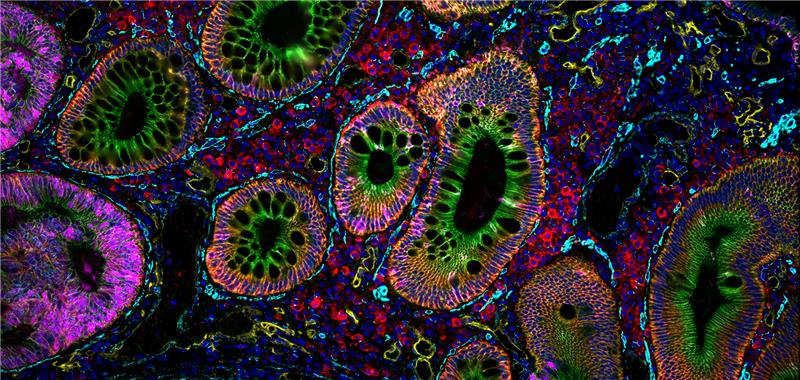
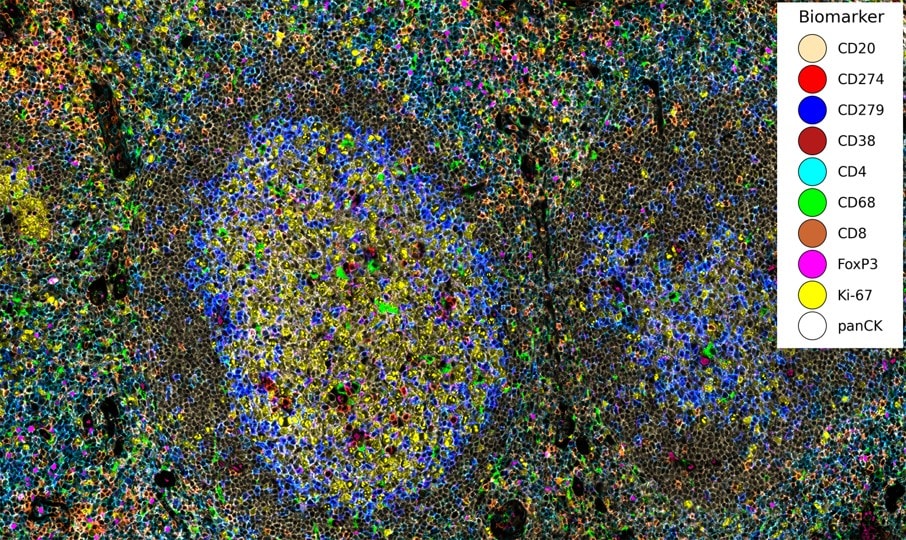
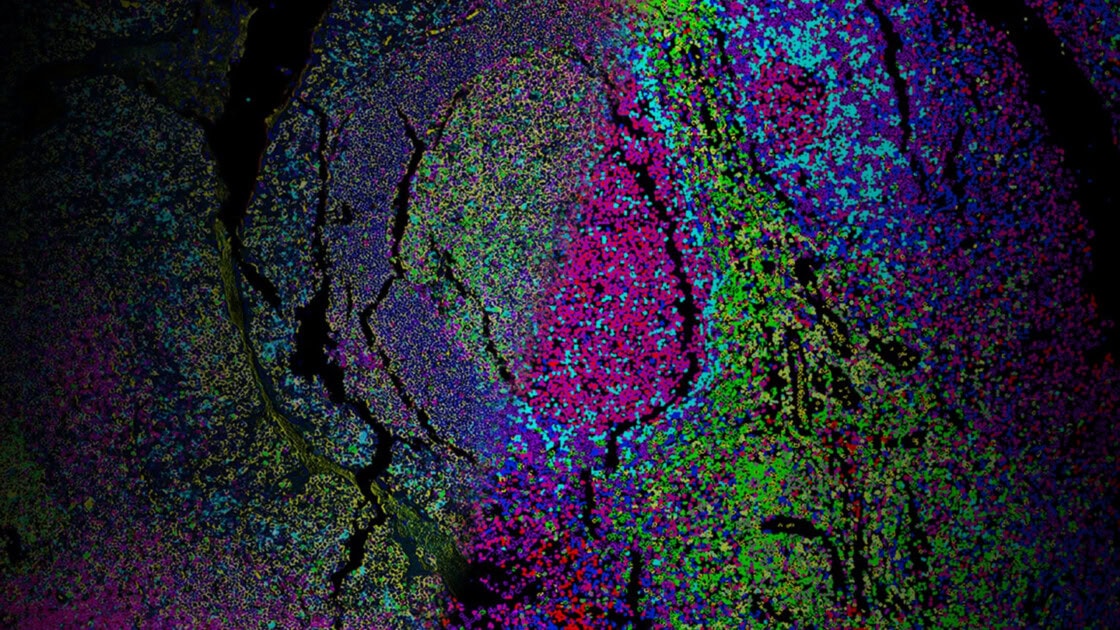
Providing a building blog for essential spatial discovery of the immune microenvironment, the Spatial Immune Profiling Assay Kit includes a panel of antibodies curated and optimized by our robust validation workflow. The panel targets key immune biomarkers and can be expanded by pairing with additional VistaPlex Multiplexing Assay Kits.
The VistaPlex Spatial Immune Profiling Assay Kit enables automated staining and imaging of 10 human FFPE samples for 17 biomarkers using the CellScape Precise Spatial Proteomics platform. An online tool is available to guide consistent and reliable execution of the multiplexed assay.

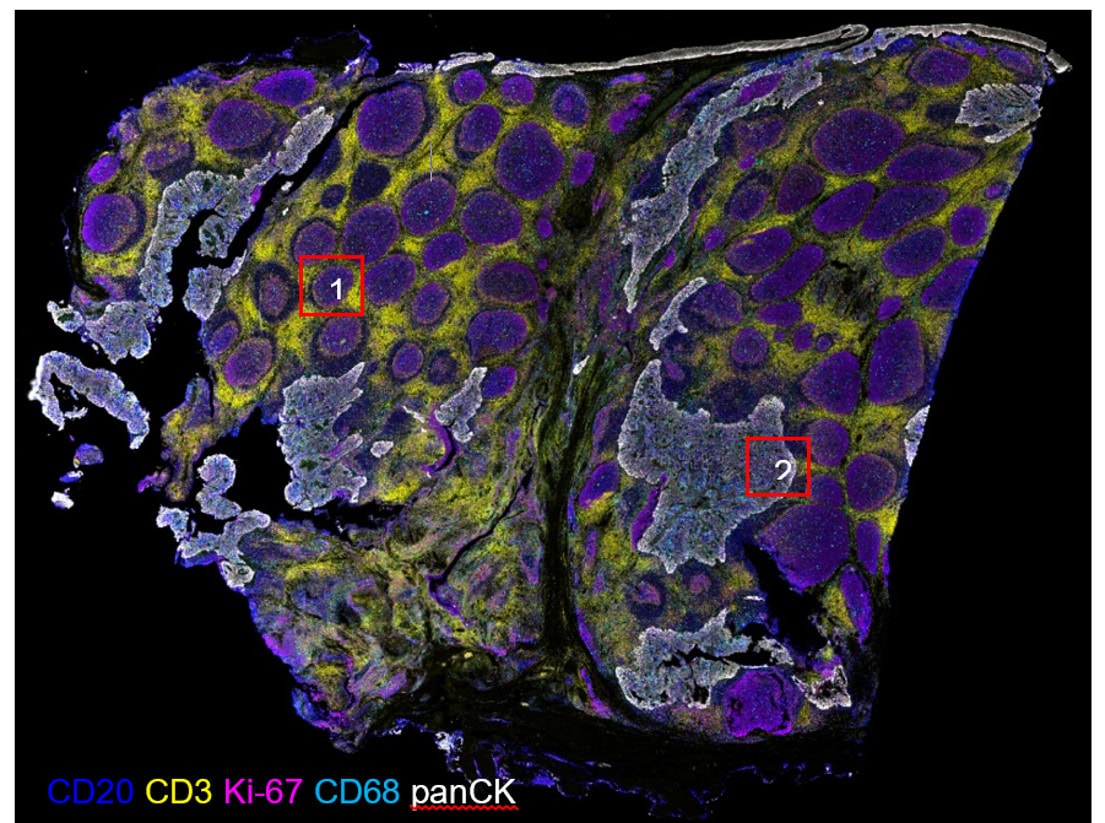
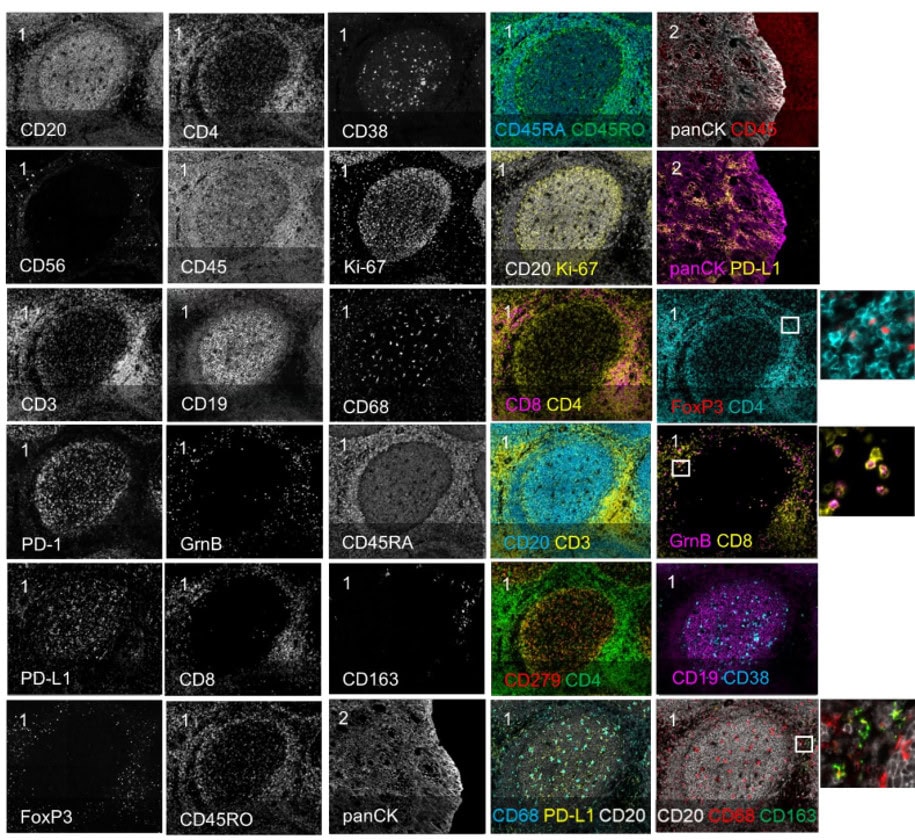
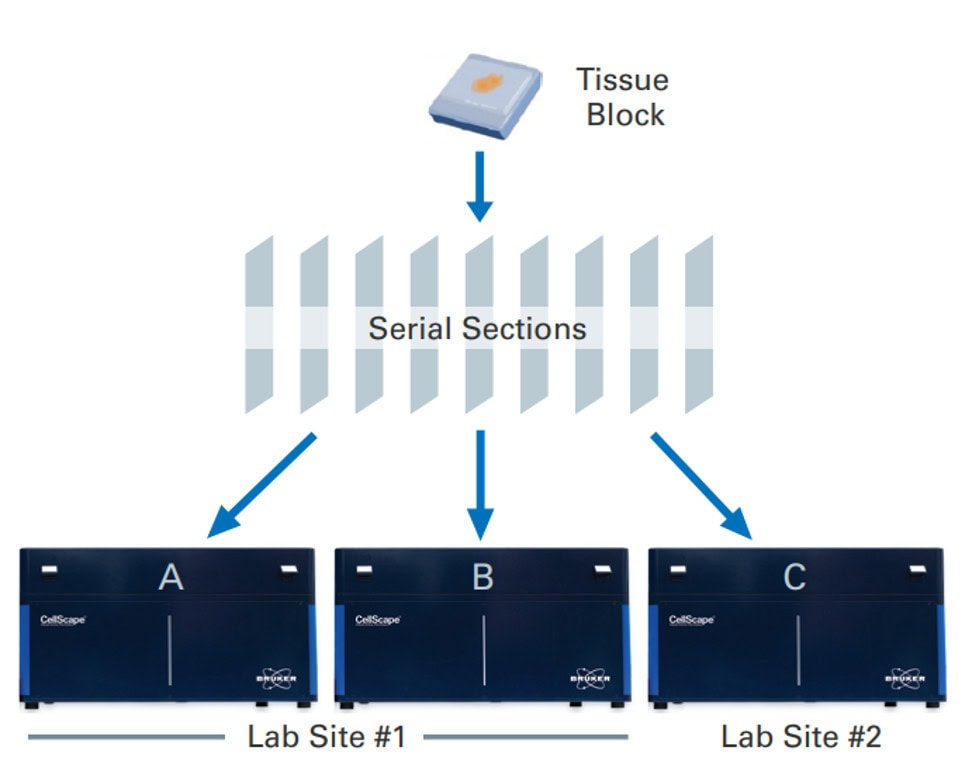
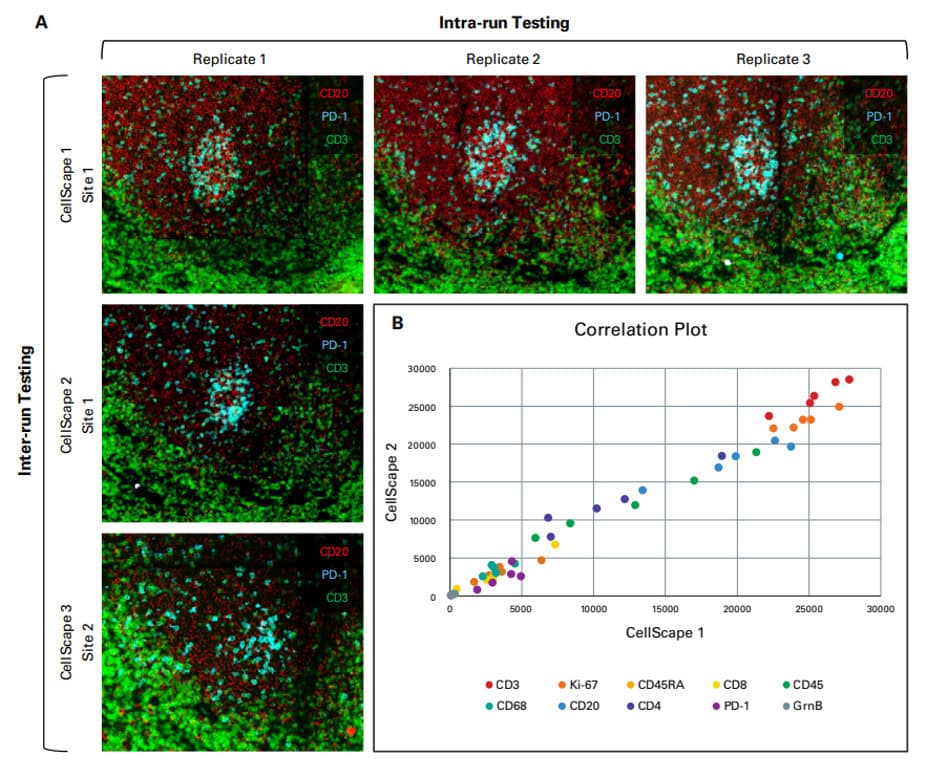
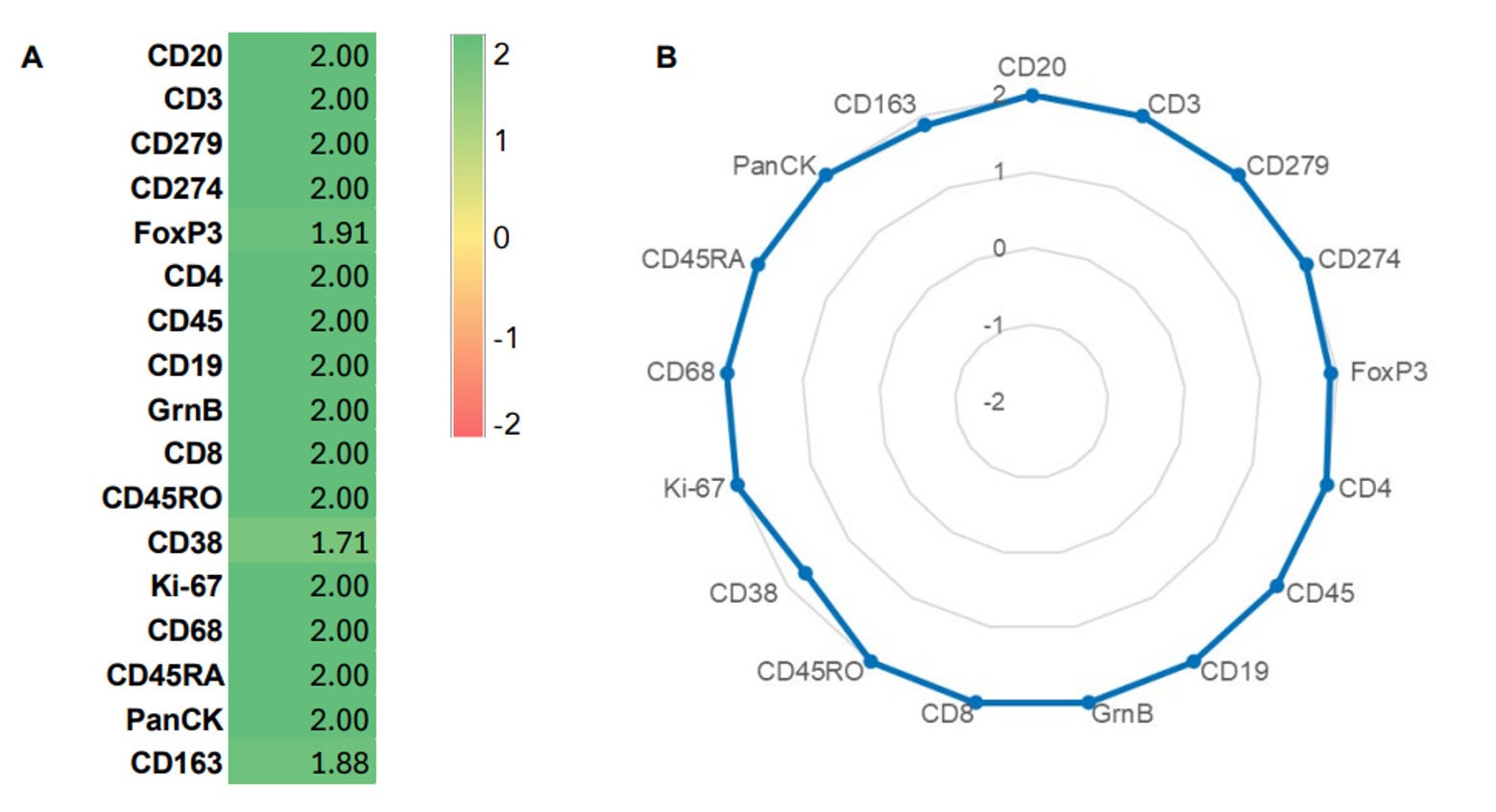
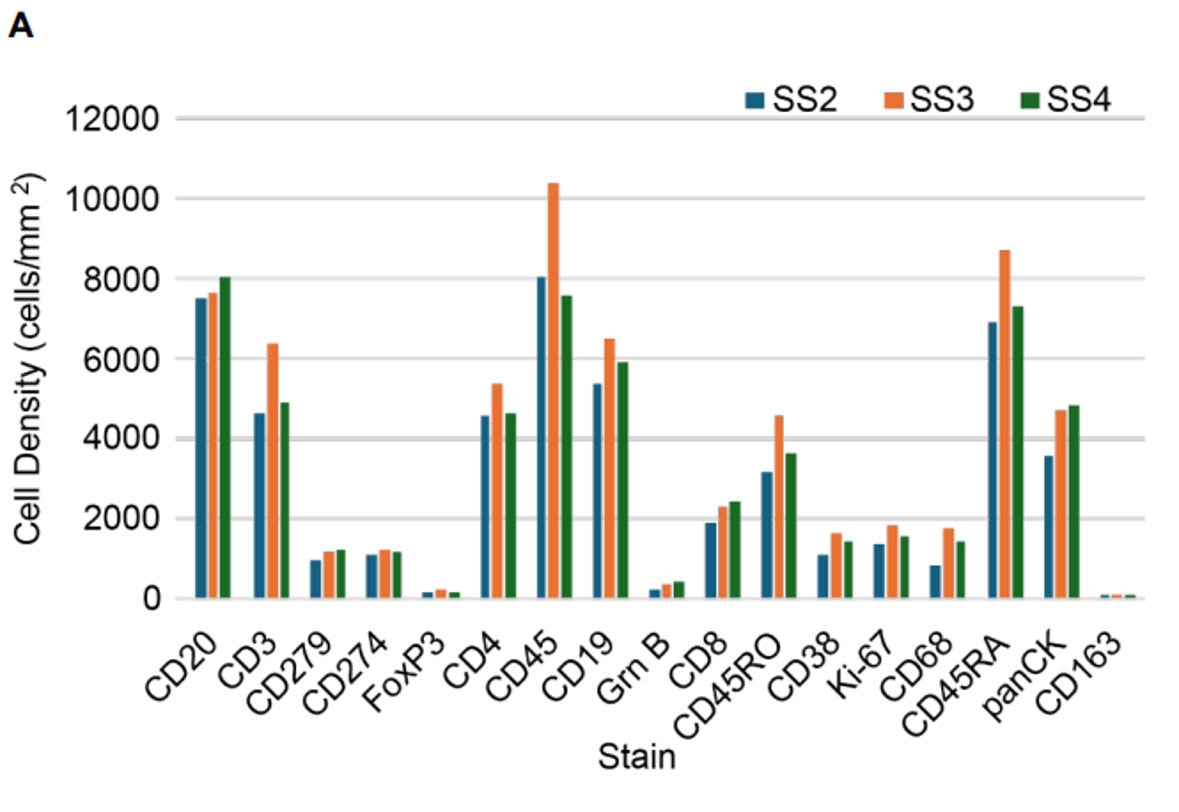
Validation is performed on human FFPE tonsil as a positive control, as well a multiple fundamental disease states.
Request a Quote
Contact our helpful experts and we’ll be in touch soon.

Related Resources